Background: Current frontline treatment of multiple myeloma (MM) often includes lenalidomide (Len) and the anti-CD38 monoclonal antibody daratumumab (Dara), with treatment administered until disease progression. There is no singular standard of care for patients (pts) refractory to these drugs at time of relapse. With the rising need for regimens that incorporate alternate agents in relapsed and/or refractory MM (RRMM), we evaluated subsequent treatment regimens that exclude Len and Dara RRMM. This study describes the most frequent real-world use of such regimens in early RRMM and patient outcomes.
Methods: A retrospective observational study of pts with RRMM was conducted using the nationwide Flatiron Health electronic health record (EHR)-derived database, a longitudinal database comprising de-identified pt-level structured and unstructured data, curated via technology-enabled abstraction (Ma et. al., 2020). Patients diagnosed with MM, ICD-9 (203.0x) or ICD-10 (C90.xx) who initiated line 2 (L2) or line 3 (L3) triplet regimens not including Len and Dara between January 2013 and December 2021 were included with follow-up through December 2022. Patients receiving one of the five most utilized (“top 5”) Len and Dara sparing L2 and L3 triplet regimens were included in the analysis. Pts could be included in both L2 and L3. Real-world overall survival (rwOS) and real-world progression free survival (rwPFS) were calculated using the Kaplan-Meier approach defined as time from initiation of L2 or L3 until death or their last visit (rwOS) and until death, last visit, Flatiron-derived progression, or initiation of next line of therapy (rwPFS), whichever occurred first.
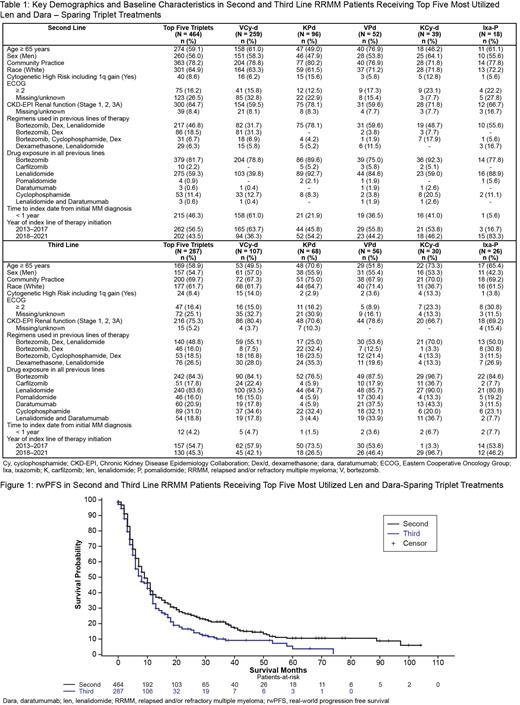
Results: Of the 6915 L2 and L3 treated RRMM pts in the database, 1023 (14.8%) received Len and Dara sparing triplet regimens with 751 (10.9%) comprising one of the top five Len and Dara sparing triplet regimens. Of those, 464 pts (6.7%) and 287 pts (4.2%) were in 2L and 3L respectively. In 2L, 259 (55.8%) were treated with bortezomib-cyclophosphamide-dexamethasone (VCy-d), 96 (20.7%) with carfilzomib-pomalidomide-dexamethasone (KPd), 52 (11.2%) with bortezomib-pomalidomide-dexamethasone (VPd), 39 (8.4%) with carfilzomib-cyclophosphamide-dexamethasone (KCy-d) and 18 (3.9%) with ixazomib-pomalidomide-dexamethasone (Ixa-Pd). Three (0.6%) pts in L2 had received frontline Dara (all 3 with Len). In 3L, 107 (37.3%) were treated with KPd, 68 (23.7%) with VCy-d, 56 (19.5%) with KCy-d, 30 (10.5%) with elotuzumab-pomalidomide-dexamethasone (Elo-Pd), and 26 (9.1%) with VPd. Of the L3 cohort, 83.6% (n=240) and 20.9% (n=60) had prior exposure to Len and Dara, respectively; 54 (18.8%) pts were double class exposed to both Len and Dara, with KPd and KCyd being the mostly used regimens (70.4%; n=38). Variation in baseline pt and disease characteristics was observed across the top five Len and Dara sparing triplet regimens in L2 and L3 and is summarized in Table 1. In L2 and L3 cohorts, overall median rwPFS was 9.0 (95% CI: 8.0-11.0) and 8.0 (95% CI: 6.0-10.0) months. Median rwOS was not reached in L2 and L3 pts, with median follow up of 25 and 18 months (Figure 1). Median rwPFS for the 54 L3 pts who were double class exposed to both Len and Dara was 6.0 (95% CI: 5.0-10.0) months. Median rwOS was not reached.
Conclusions: With the evolution of MM practice, the proportion of RRMM pts exposed and refractory to Dara and Len is expected to continue growing. Efficacious Dara and Len sparing regimens with demonstrated real-world utility, such as KPd and KCy-d, are increasingly needed, especially beginning at first relapse of MM.
Disclosures
Braunlin:Amgen Inc.: Current Employment, Current equity holder in publicly-traded company. Najdi:Amgen Inc.: Current Employment, Current holder of stock options in a privately-held company. Arora:Amgen Inc.: Current Employment, Current equity holder in publicly-traded company. Kimball:Amgen Inc.: Current Employment, Current equity holder in publicly-traded company. McFadden:Amgen: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal